TMS THERAPY- A diagnosis is the first step in determining the necessary treatment for any patient’s recovery. A diagnosis can include medications, surgery, therapy, and more.
An accurate and ideal diagnosis is essential in preventing wasting valuable time during an incorrect treatment.
Each patient’s qualities and needs are crucial in helping to determine the correct diagnosis, and many factors are involved.
Carrying out a general clinical interview, a psychological examination, and a physical diagnostic test will help to understand the clinical and family history of the patient.
Transcranial Magnetic Stimulation bases its ideal on two fundamental principles of physics: electricity and magnetism.
TMS Therapy manages to fuse these two principles and has been studied primarily as a treatment for depression, OCD, and anxiety.
TMS, also called Transcranial Magnetic Stimulation, is an alternative to antidepressant medication and a way to treat Major Depressive Disorder (MDD) and treatment-resistant depression.
Many medical studies have shown that TMS has proven to be the most effective therapy for improving symptoms of major depression.
The magnetic pulses generated by the TMS Therapy induce small electric currents that change the firing patterns of neurons. These magnetic pulses alter dysfunctional brain patterns that are associated with depression.
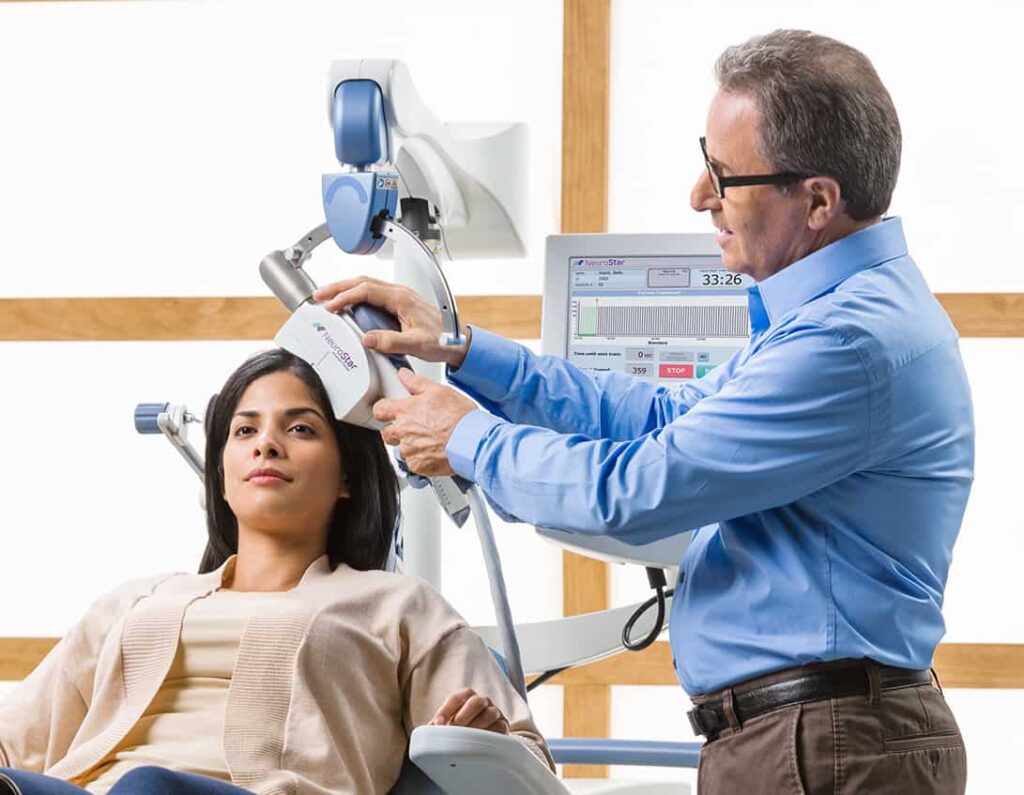
How to determine whether or not you are a candidate for a TMS therapy treatment?
TMS is often a treatment that can help when other treatment options are unsuccessful. It is also an essential option because it is non-invasive.
To be eligible for TMS treatment, a person struggling with a mental health condition must meet specific requirements.
NeuroStar TMS Therapy is not an option for patients with non-removable conductive metal or stimulator implants in or near the head or patients with active or inactive implants such as deep brain stimulators, cochlear implants, and vagus nerve stimulators.
Some factors can help identify a beneficial treatment during the sessions.
Most patients with a major depressive disorder, proven to be treatment-resistant, are approved to start TMS therapy.
Still, you might not be approved for treatment if you:
Patients should notify their doctor if they experience worsening depression symptoms, signs or symptoms of suicidal behavior, and unusual behavior. Family members and support individuals should also be aware of the need to observe patients and notify their treatment provider if symptoms worsen.
Other criteria also involve any possibility of being a TMS Therapy candidate.
For example, many US insurance providers define Major Depressive Disorder (MDD) treatment coverage approval if:
– The patient is currently battling a depressive episode, with the episode’s documented duration dates.
– Within the current depressive episode, the patient has undergone failed antidepressant trials.
– The patient demonstrates that a trial of evidence-based psychotherapy was also ineffective (With characteristics and the name of the professional that provided it).
– In cases where the patient has undergone previous TMS or ECT treatment, they will need to present the treatment’s details, with dates and scores.
Every patient responds differently to TMS therapy, and the measurement of its success is a deeply personal one.
The most common side effect of TMS Treatment is generally mild-to-moderate pain or discomfort at or near the treatment area during the session.
When this occurs, it is temporary and typically occurs only during the first week of treatment.
There are no effects on alertness or understanding; patients treated with NeuroStar TMS Therapy can drive themselves to and from their treatment sessions.
Because it is not a depression drug, NeuroStar TMS Therapy doesn’t have the side effects often associated with antidepressant medication.
Talking with your doctor to determine whether TMS is right for you or not will always be the first option. Most insurance carriers will provide coverage for TMS only after patients have undergone trials of psychotherapy and medication without success.
NeuroStar TMS Therapy has performed more than two million treatments. NeuroStar TMS Therapy is an FDA-cleared safe, and effective non-drug depression treatment for patients who are not satisfied with the results of standard drug therapy. In addition, this novel treatment option provides benefits without the side effects often associated with antidepressant medication.
At Long Island Neurocare, our experienced team will work with your health insurance company to determine your benefits, coverage, and out-of-pocket costs. We will discuss your insurance coverage, out-of-pocket costs, and reimbursement during your visit. We look forward to assisting you with the insurance process.