TMS Therapy is an outpatient, in-clinic treatment, where patients are awake and alert during all treatment sessions, and no medications are used.
During treatment, the patient hears a clicking sound and feels a tapping sensation on the head. The most common side effect is generally mild-to-moderate pain or discomfort at or near the treatment area during the session. When this occurs, it is temporary, and typically occurs only during the first week of treatment.
There are no effects on alertness or understanding; patients being treated with TMS Therapy can drive themselves to and from their treatment sessions.
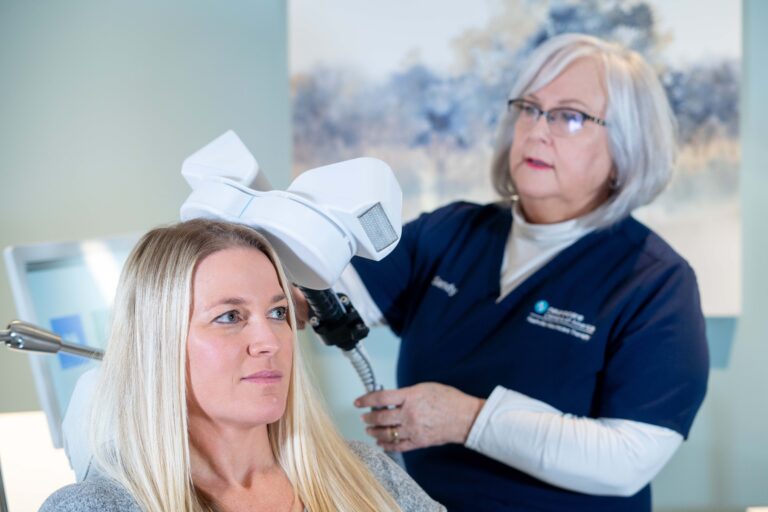
Step #1: The patient reclines comfortably in the treatment chair, awake and alert, and can speak with the clinical operator whenever necessary
Step #2: A small curved device containing the magnetic coil rests lightly on the patient’s head
Step #3: The device delivers focused magnetic stimulation directly to the target areas of the brain
Step #4: The patient can immediately resume normal activities because there is no effect on alertness or understanding
Because it is not a depression drug, TMS Therapy doesn’t have the side effects that are often associated with antidepressant medication.4,5 More than two million treatments have been performed with TMS Therapy.
TMS Therapy is free of systemic side effects typically experienced with antidepressant medications.4,5
In clinical trials, fewer than 5% of patients discontinued treatment with TMS Therapy due to adverse events. Other side effects (occurring in ≥5% of patients and twice the rate of placebo) include eye pain, toothache, muscle twitch, facial pain and/or pain of the skin. There is a rare risk of seizure with TMS Therapy (0.1% of patients). There is no adverse effect on cognition.8
Patients should notify their doctor if they experience worsening depression symptoms, signs or symptoms of suicidal behavior and/or unusual behavior. Family members and support individuals should also be aware of the need to observe patients and notify their treatment provider if symptoms worsen.
TMS Therapy should not be used with patients who have non-removable conductive metal or stimulator implants in or near the head or patients who have active or inactive implants such as deep brain stimulators, cochlear implants, and vagus nerve stimulators.
Copyright © 2025 | All Rights Reserved